Iridium(III) chloride
 α-IrCl3
| |
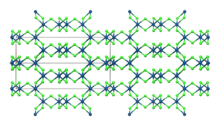 β-IrCl3
| |
 Iridium(III) chloride trihydrate
| |
| Names | |
|---|---|
| Other names
Iridium trichloride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.028 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| IrCl3 | |
| Molar mass | 298.58 g/mol (anhydrous)
316.60 g/mol (hydrate) |
| Appearance | brown solid (α-anhydrous) red solid (β-anhydrous) dark green solid (trihydrate) |
| Density | 5.30 g/cm3, solid[1] |
| Melting point | 763 °C (1,405 °F; 1,036 K)[1][2] (decomposes) |
| insoluble (anhydrous IrCl3), soluble (hydrated derivative)[1] | |
| Solubility | Insoluble in HCl and alkanes[1] |
| −14.4·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-257 kJ/mol |
| Hazards | |
| GHS labelling:[3] | |
 
| |
| Warning | |
| H302, H411 | |
| Flash point | non-flammable |
| Related compounds | |
Other cations
|
Rhodium(III) chloride |
Related compounds
|
Platinum(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.[4]
Preparation
[edit]Iridium is separated from the other platinum group metals as crystalline ammonium hexachloroiridate, (NH4)2[IrCl6], which can be reduced to iridium metal in a stream of hydrogen. The spongy Ir thus produced reacts with chlorine at 650 °C to give iridium(III) chloride.[5]
Hydrated iridium trichloride is obtained by heating hydrated iridium(III) oxide with hydrochloric acid.[6]
Structure
[edit]Like the related rhodium compound, IrCl3 adopts the structure seen for aluminium chloride.[6] This is the monoclinic α polymorph.[7] A rhombohedral β polymorph also exists. Both polymorphs have effectively the same anion lattice but differ in the octahedral interstices the iridium ions occupy.[8] The α polymorph converts to the β polymorph when heated to around 650 °C.[4]
| Compound | α-IrCl3[7] | β-IrCl3[8] |
|---|---|---|
| Crystal Structure | Monoclinic | Orthorhombic |
| Space Group | C2/m | Fddd |
| Lattice constant a (Å) | 5.99 | 6.95 |
| Lattice constant b (Å) | 10.37 | 9.81 |
| Lattice constant c (Å) | 5.99 | 20.82 |
| β | 109.4° | |
| Calculated density (g/cm3) | 5.33 | 5.34 |
The structure of the trihydrate has not been elcudated yet.
Reactions and uses
[edit]Industrially, most iridium complexes are generated from ammonium hexachloroiridate or the related chloroiridic acid (H2IrCl6). The Cativa process, source of most of the world's acetic acid relies on such catalysts.
Hydrated iridium(III) chloride is used in the for the preparation of other iridium complexes such as Vaska's complex, trans-[IrCl(CO)(PPh3)2].[9] With the presence of the chloride anion, it forms hexachloroiridate(III), and produces hexachloroiridate(IV) in aqua regia. The trihydrate react with ammonia to form ammine complexes, such as pentaamminechloroiridium(III) chloride, formulated [IrCl(NH3)5]Cl2. It also reacts with concentrated ammonium hydroxide at 150 °C to form the fully ammoniated complex, [Ir(NH3)6]Cl3. The hydrate can also form complexes upon reaction with bipyridine, acetonitrile, and pyridine.[4]
Alkene complexes such as cyclooctadiene iridium chloride dimer[10][11] and chlorobis(cyclooctene)iridium dimer[11][10] can also be prepared by heating the hydrate with the appropriate alkene in water/alcohol mixtures.
Decomposition
[edit]The trihydrate decomposes to the anhydrous form at 200 °C, which then oxidizes in air at 763 °C to iridium(IV) oxide, which then decomposes to iridium metal at 1070 °C. However, under hydrogen, it is directly reduced at 190 °C to iridium metal:[2][12][13]
- 2 IrCl3 + 3 H2 → 2 Ir + 6 HCl
Safety
[edit]Iridium(III) chloride is not listed under Annex I of Directive 67/548/EEC, but is listed in the inventory of the Toxic Substances Control Act (TSCA). It is also known as a mild skin and eye-irritating agent.[14]
References
[edit]- ^ a b c d Haynes, William, ed. (2014). CRC Handbook of Chemistry and Physics. CRC Press. p. 4-68. ISBN 9781482208689.
- ^ a b A.E. Newkirk; D.W. McKee (1968). "Thermal decomposition of rhodium, iridium, and ruthenium chlorides". Journal of Catalysis. 11 (4): 370–377. doi:10.1016/0021-9517(68)90061-4.
- ^ "C&L Inventory". echa.europa.eu. Retrieved 23 December 2021.
- ^ a b c C. E. Housecroft and A. G. Sharpe Inorganic Chemistry, p. 849.
- ^ Hermann Renner (2018). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry: 1–73. doi:10.1002/9783527306732.a21_075.pub2. ISBN 9783527303854. S2CID 94472506.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Brodersen, K.; Moers, F.; Schnering, H. G. (1965). "Zur Struktur des Iridium(III)- und des Ruthenium(III)-chlorids". Naturwissenschaften. 52 (9): 205–206. doi:10.1007/BF00626459. S2CID 43351743.
- ^ a b Meisel, A.; Leonhardt, G. (1965). "Die Kristallstruktur von β-Iridium(III)-Chlorid". Z. anorg. allg. Chem. 339 (1–2): 57–66. doi:10.1002/zaac.19653390109.
- ^ Vaska, L.; & DiLuzio, J. W. (1961) J. Am. Chem. Soc. 83:2784. Girolami, G.S.; Rauchfuss, T.B.; Angelici, R.J. (1999). Synthesis and Technique in Inorganic Chemistry (3rd Edn.). Sausalito: University Science Books.
- ^ a b Winkhaus, G.; & Singer, H. (1966). Iridium(I)-Olefinkomplexe. Chem. Ber. 99:3610–18.
- ^ a b Herde, J. L.; Lambert, J. C.; & Senoff, C. V. (1974). Cyclooctene and 1,5-Cyclooctadiene Complexes of Iridium(I). Inorg. Synth. 1974, volume 15, pages 18–20. doi:10.1002/9780470132463.ch5.
- ^ W. B. Rowston; J. M. Ottaway (1979). "Determination of noble metals by carbon furnace atomic-absorption spectrometry. Part 1. Atom formation processes". Analyst. 104 (1240): 645–659. Bibcode:1979Ana...104..645R. doi:10.1039/AN9790400645.
- ^ R. K. Kawar; P. S. Chigare; P. S. Patil (2003). "Substrate temperature dependent structural, optical and electrical properties of spray deposited iridium oxide thin films". Applied Surface Science. 206 (1–4): 90–101. Bibcode:2003ApSS..206...90K. doi:10.1016/S0169-4332(02)01191-1.
- ^ Mager Stellman, J. (1998). "Iridium". Encyclopaedia of Occupational Health and Safety. International Labour Organization. pp. 63.19. ISBN 978-92-2-109816-4. OCLC 35279504.
